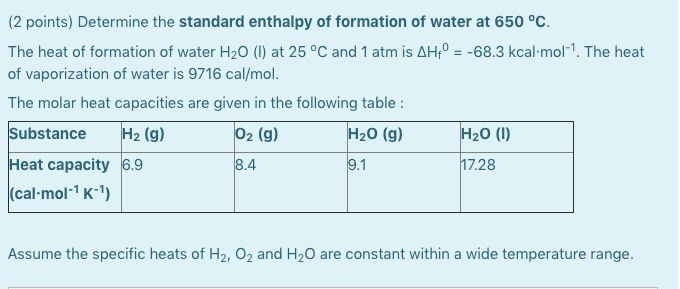
Calculate change in enthalpy when 2 moles of liquid water at 1 bar and `100^(@)` C is coverted into - YouTube

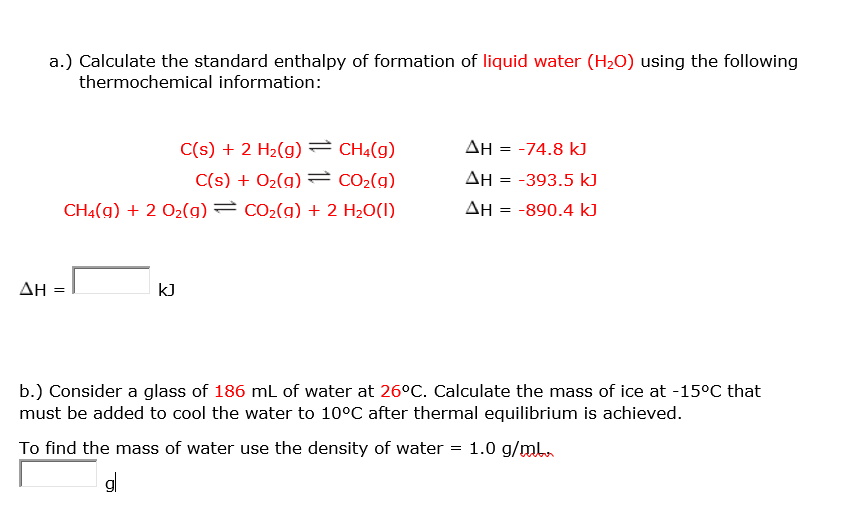
Calculate the enthalpy change of freezing of `1.0` mol of water at `10^()C` to ice at `-10^()C, ... - YouTube
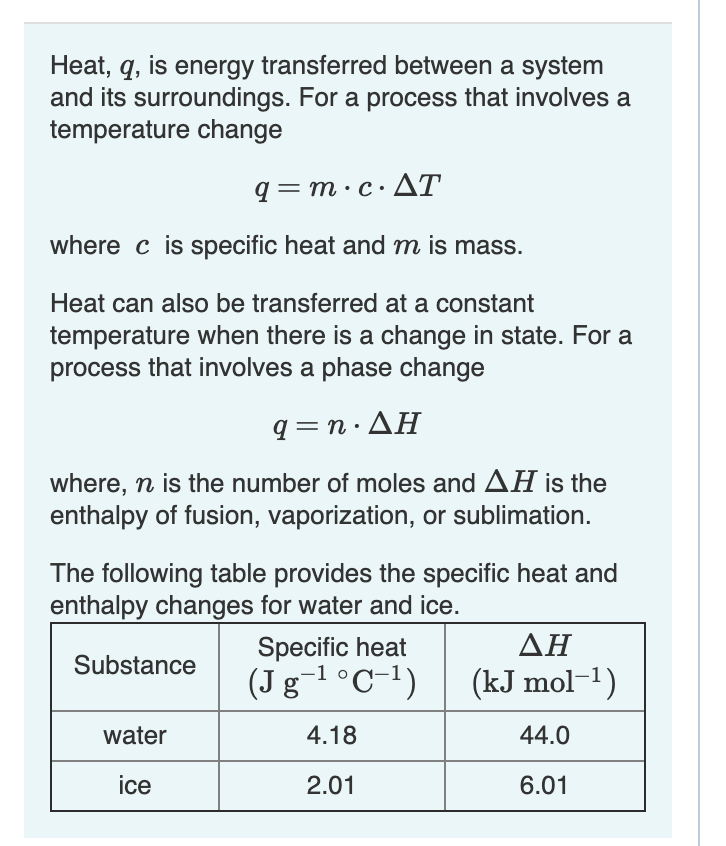
![Calculate the enthalpy change of freezing of 1.0 mol of water at 10^(@)C to ice at -10^(@)C, Delta(fus)H=6.03 kJ mol^(-1) at 0^(@)C. C(P)[H(2)O(l)]=75.3 J mol^(-1) K^(-1) C(P)[H(2)O(s)]=36.8 J mol^(-1) K^(-1) Calculate the enthalpy change of freezing of 1.0 mol of water at 10^(@)C to ice at -10^(@)C, Delta(fus)H=6.03 kJ mol^(-1) at 0^(@)C. C(P)[H(2)O(l)]=75.3 J mol^(-1) K^(-1) C(P)[H(2)O(s)]=36.8 J mol^(-1) K^(-1)](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/69096014_web.png)
Calculate the enthalpy change of freezing of 1.0 mol of water at 10^(@)C to ice at -10^(@)C, Delta(fus)H=6.03 kJ mol^(-1) at 0^(@)C. C(P)[H(2)O(l)]=75.3 J mol^(-1) K^(-1) C(P)[H(2)O(s)]=36.8 J mol^(-1) K^(-1)